welcome to CARTI clinical research
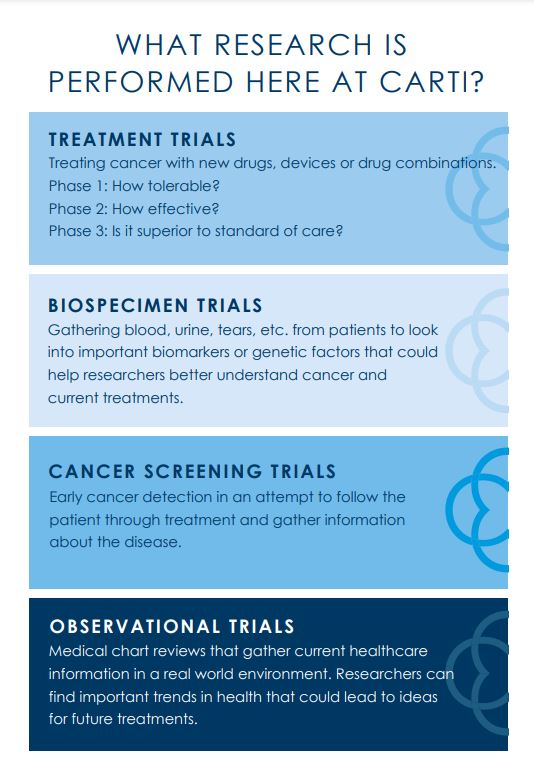
Our research team is currently working with pharmaceutical companies on over 35 clinical trials to bring advancement to oncology medicine. Clinical trials examine the safety and efficacy of new treatments, reduce the time between treatment and response evaluation, improve supportive care medications, monitor treatments in the real world setting and provide biological samples to develop tests for early cancer detection. Here at CARTI we are proud to offer front-line research to our patients. Our goal is to work toward innovation and improvement in standard of care. With a mission of providing the most leading-edge cancer care available, CARTI’s medical team actively participates in research projects that will impact the treatment options we are able to provide our current and future patients.
how do I participate in a clinical trial?
The purpose of our research is to engage in research-driven patient care by offering the most effective new treatment options in order to extend survival, improve quality of life and advance the knowledge of cancer treatment. If you are interested in participating in a clinical trial, ask your physician if you qualify. To make our clinical trials available to all CARTI patients, all of our physicians serve as sub-investigators; this means that your treating physician can enroll you onto a clinical trial if you are eligible. Please refer to the list below to view our active studies!
Participation in research is always voluntary. For further information on clinical trials, please visit http://www.clinicaltrials.gov.
If you have any additional questions for or about CARTI’s Research Team, please call 501.906.4199 or email Research@CARTI.com.
BREAST CANCER TRIALS

HER2Climb-05 (SGNTUC-028)
Sponsor: Seagen
Principal Investigator: Dr. Sam Makhoul
A Randomized, Double-Blind, Phase 3 study of Tucatinib or Placebo in Combination With Trastuzumab And Pertuzumab As Maintenance Therapy For Metastatic HER2+ Breast Cancer

SERENA-6 (D8534C00001)
Sponsor: AstraZeneca
Principal Investigator: Dr. Sam Makhoul
A Phase III, Double-blind, Randomised Study to Assess Switching to AZD9833 + CDK4/6 Inhibitor vs Continuing AI + CDK4/6 Inhibitor in HR+/HER2- MBC Patients with ctDNA Detectable ESR1 Mutation Without Disease Progression During 1L Treatment

Tropion Breast-03 (D926XC00001)
Sponsor: AstraZeneca
Principal Investigator: Dr. Sam Makhoul
A Study of Dato-DXd With or Without Durvalumab Versus Investigator’s Choice of Therapy in Patients With Stage I-III Triple-negative Breast Cancer Without Pathological Complete Response Following Neoadjuvant Therapy

VIKTORIA (Celc-G-301)
Sponsor: Celcuity, Inc.
Principal Investigator: Dr. Sam Makhoul
A Phase 3, Open-Label, Randomized, Two-Part Study Comparing Gedatolisib in Combination with Palbociclib and Fulvestrant to Standard-of-Care Therapies in Patients with HR-Positive, HER2-Negative Advanced Breast Cancer Previously Treated with a CDK4/6 Inhibitor in Combination with Non-Steroidal Aromatase Inhibitor Therapy
COLORECTAL CANCER TRIALS

ONSEMBLE (CRDF-003)
Sponsor: Cardiff Oncology
Principal Investigator: Dr. Lawrence Mendelsohn
A Phase 2, Randomized, Open-label Study of Onvansertib in Combination with FOLFIRI and Bevacizumab Versus FOLFIRI and Bevacizumab for Second Line Treatment of Metastatic Colorectal Cancer in Patients with a KRAS or NRAS Mutation
LUNG CANCER TRIALS

AVANZAR (D926NC00001)
Sponsor: AstraZeneca
Principal Investigator: Dr. Ryan Hall
A Phase III, Randomised, Open-label, Multicentre, Global Study of Datopotamab Deruxtecan (Dato-DXd) in Combination With Durvalumab and Carboplatin Versus Pembrolizumab in Combination With Platinum-based Chemotherapy for the First-line Treatment of Patients With Locally Advanced or Metastatic NSCLC Without Actionable Genomic Alterations

MERCK Keynote 867 (3475-867-05)
Sponsor: Merck & Co.
Principal Investigator: Dr. Sam Makhoul
A Phase 3, Randomized, Placebo-Controlled Clinical Study to Evaluate the Safety and Efficacy of Stereotactic Body Radiotherapy (SBRT) with or without Pembrolizumab (MK-3475) in Participants with Unresected Stage I or II Non-Small Cell Lung Cancer (NSCLC)

MERCK MK2870-023 : COMING SOON
Sponsor: Merck & Co.
Principal Investigator: Dr. Ryan Hall
A Phase 3 Study of Pembrolizumab in Combination With Carboplatin/Taxane (Paclitaxel or Nab-paclitaxel) followed by Pembrolizumab With or Without Maintenance MK-2870 in the First-line Treatment of Metastatic Squamous Non-small Cell Lung Cancer
PROSTATE CANCER TRIALS

EvoPAR (D9723C00001)
Sponsor: AstraZeneca
Principal Investigator: Dr. Keith Mooney
A Randomized, 2-cohort, Double-blind, Placebo controlled, Phase III Study of Saruparib (AZD5305) in Combination with Physician’s Choice New Hormonal Agents in Patients with HRRm and non-HRRm Metastatic Castration-Sensitive Prostate Cancer
HEM MALIGNANCY TRIALS

ESCALADE (D8227C00001)
Sponsor: AstraZeneca
Principal Investigator: Dr. Appalanaidu Sasapu
A Phase 3, Randomized, Double-Blind, Placebo-Controlled Study of Acalabrutinib in Combination with Rituximab, Cyclophosphamide, Doxorubicin, Vincristine, and Prednisone (R-CHOP) in Subjects ≤75 Years with Previously Untreated Non-Germinal Center Diffuse Large B-Cell Lymphoma
SOLID TUMOR TRIALS

CASE (R2810-ONC-1806)
Sponsor: Regeneron Pharmaceuticals, Inc.
Principal Investigator: Dr. Rhonda Gentry
CemiplimAb Survivorship Epidemiology (CASE) Study

IGNYTE (RPL-001-16)
Sponsor: Replimune, Inc.
Principal Investigator: Dr. Sam Makhoul
An Open-Label, Multicenter, Phase 1/2 Study of RP1 as a Single Agent and in Combination with PD1 Blockade in Patients with Solid Tumors

PRO-ACC1 (C3651003)
Sponsor: Pfizer
Principal Investigator: Dr. Ryan Hall
A Phase 2, Randomized, Double-Blind, Placebo-Controlled Study To Investigate the Efficacy, Safety and Tolerability of Ponsegromab in Patients With Cancer, Cachexia, and Elevated Concentrations of GDF-15, Followed By an Optional Open-Label Treatment Period
INVESTIGATIONAL DEVICE TRIALS

INSTYLLA (INY-P-20-001)
Sponsor: Instylla, Inc.
Principal Investigator: Dr. Edgar St. Amour
Randomized Multi-Center, Subject and Evaluator Blinded, Parallel-Group Study to Evaluate the Safety and Effectiveness of the Instylla Hydrogel Embolic System (HES) Compared with Standard of Care Transcatheter Arterial Embolization (TAE) / Transcatheter Arterial Chemoembolization (cTACE) for Vascular Occlusion of Hypervascular Tumors; A Pivotal Study. Investigational Device: Instylla Hydrogel Embolic System (HES) including Instylla Delivery Kit and Instylla Microcatheter
CARTI is the #1 site for patient enrollment in the world for Instylla.

iPredict (IAB-CD8-203)
Sponsor: ImaginAb, Inc.
Principal Investigator: Dr. David Hays
A Phase IIB, Open Label, Study of Zirconium Zr 89 Crefmirlimab Berdoxam PET/CT in Subjects with Selected Advanced or Metastatic Malignancies including Melanoma, Merkel Cell, Renal Cell and Non-Small Cell Lung Cancers, Scheduled to Receive Standard-of-Care Immunotherapy (IOT) as a Single Agent or Combination, to Predict Response to Therapy. Device/Product Name: zirconium Zr 89 crefmirlimab berdoxam

READY (CUSA-081-HEM-01)
Sponsor: Chiesi
Principal Investigator: Dr. Ryan Hall
A Phase 3, Randomized, Double-Blind, Active and Placebo-Controlled Study on the use of CUSA-081 for Dysfunctional Central Venous Access Devices (CVADs). Device: CVAD/reteplase
BIOSPECIMEN TRIALS

CYBRID-02 (BSK22-562)
Sponsor: Elephas Biosciences Corporation
CRO: Hoosier Cancer Research Network (HCRN)
Principal Investigator: Dr. David Hays
Observational basket trial to collect tissue to train and validate a live tumor diagnostic platform


iPROCESS
Sponsor: Bluestar Genomics & Iprocess Global Research Inc.
Principal Investigator: Dr. Kamal Patel
Collection and Distribution of Biofluids for Research Purposes Cancer Types Protocol

MT Group
Sponsor: The MT Group
Principal Investigator: Dr. Kamal Patel
Translational Medicine: Discovery and Evaluation of Biomarkers/Pharmacogenomics
for the Diagnosis and Personalized Management of Patients

NAMIDA
Sponsor: Namida Lab, Inc. & Dr. Ravishankar.
Principal Investigator: Dr. Sam Makhoul
Investigating the Diagnostic Potential of Tear Proteins in Breast, Ovarian, Prostate, Melanoma, Pancreatic, and Colon Cancer. Open Cohort: Ovarian
CARTI Partners with Namida Lab to Study Proteins In Cancer Patients’ Tears
RESEARCH NEWS AND EVENTS
Learn what research and clinical trials can mean for you from our Medical Director of Research, Dr. Sam Makhoul.
Watch this video to hear about the inspirational cancer journey of Carol Wadley and the role research played in her care.
ABSTRACTS AND ARTICLES BY CARTI PHYSICIANS
- Phase IIIb Safety and Efficacy of Intravenous Nepa for Prevention of Chemotherapy-Induced Nausea and Vomiting (CINV) in Patients with Breast Cancer Receiving Initial and Repeat Cycles of Anthracycline and Cyclophosphamide (AC) Chemotherapy. Kamal Patel, M.D., The Oncologist.
- Mortality and Causes of Death for Patients with Polycythemia Vera: Analysis of the REVEAL Prospective, Observational Study. Kamal Patel, M.D., American Society of Hematology.
- Demographics, Prior Therapies and Reasons for Cemiplimab Treatment: Prospective CemiplimAb-rwlc Survivorship and Epidemioogy (C.A.S.E.) Study in Patients with Advanced Cutaneous Squamous Cell Carcinoma. Rhonda Gentry, M.D.
- The Right to Try, Kamal Patel, M.D., The Healthcare Journal of Arkansas.

